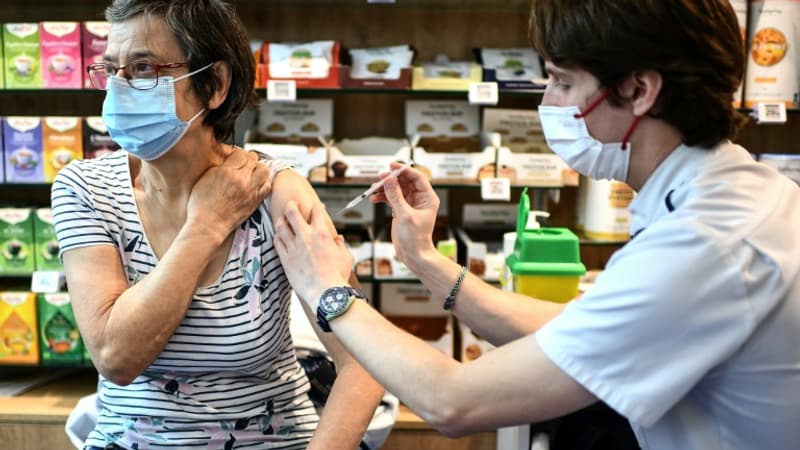
The risk of suffering from menstrual disorders increases slightly during the three months following the administration of an anti-Covid messenger RNA vaccine, suggests a study published this Wednesday, January 24, by the French health authorities, while such a relationship continues to be debated in the community. scientific. .
The study “shows a 20% increase in the risk of heavy menstrual bleeding requiring hospital treatment within 1 to 3 months”, after having received a Pfizer or Moderna vaccine for the first time, summarizes the organization Epi-Phare in a press release.
Epi-Phare is an organization that brings together the Medicines Authority (ANSM) and Social Security, responsible in particular for protecting people in terms of health.
Contradictory studies
Since the start of anti-Covid vaccination campaigns, almost two and a half years ago, many women have reported alterations in their menstrual cycles.
Based on these statements, the European Medicines Agency (EMA) ended up including the presence of heavy menstrual bleeding as a possible side effect of the messenger RNA vaccines, those from Pfizer/BioNTech and Moderna. However, studies on the subject remain contradictory.
The Epi-Phare one gives arguments to those who are in favor of the existence of such a link.
The researchers identified the vaccination status of several thousand women hospitalized for heavy menstrual bleeding in 2021 and 2022. They compared their status to a control group of women who had not been treated for this reason.
Ultimately, they conclude that the risk of suffering from said menstrual disorder is slightly higher the first time a woman receives a Moderna or Pfizer vaccine, each administered in two successive doses. The risk lasts three months and then, even after a subsequent booster dose, disappears.
A question of methodology?
These results contrast with those of a large-scale study conducted in Sweden and published in spring 2023 in the BMJ. It had been estimated that there was no strong evidence of a link between Covid vaccination and menstrual disorders.
Questioned by Agence France Presse about these different conclusions, Epi-Phare researchers cited differences in methodology. They note in particular that the Swedish study took into account a period of risk that began just over a week after patients were vaccinated.
This choice “could have masked a possible increased risk that would occur a little later,” explained epidemiologist Rosemary Dray-Spira, who supervised the French study, who chose to wait a month after the first vaccine dose.
Source: BFM TV