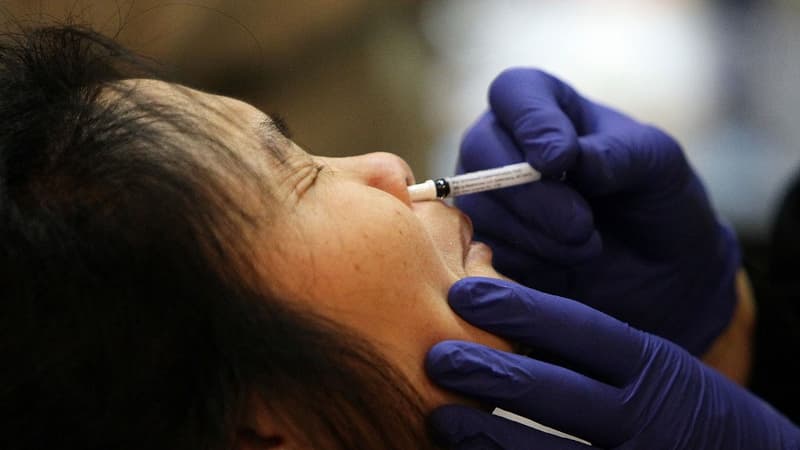
A new step forward in the fight against Covid-19. Intranasal vaccines, which generate an immune response in the respiratory mucosa, allow “to put a first line of defense, where the virus enters and does a lot of damage,” said the director of the WHO Emergency Response Situation Management Program . Officer, Dr. Mike Ryan, at a press conference.
This type of vaccine “could give us a better chance of controlling covid in the long term,” argued Dr. Ryan.
Patience
The WHO promotes the development of second and third generation vaccines “that we may need to deal with the last phase of Covid and other viruses that can attack the respiratory tract,” said the director.
However, the UN agency will wait until it has enough data on these vaccines before evaluating and possibly officially approving them.
“We are looking forward to seeing the data to see how we can integrate it into the Covid-19 response,” said WHO director of epidemic control Maria Van Kerkhove.
India approved its first intranasal vaccine against Covid-19 on Tuesday, giving a new impetus to its gigantic vaccination campaign.
The vaccine, recommended as the first dose for the adult population, has received emergency approval from India’s drug regulatory authority.
Approved in China
On Sunday, China approved the world’s first inhalable vaccine, administered as an emergency booster dose, nasally, through a sprayer, and developed by Chinese manufacturer CanSino Biologics.
For his part, WHO Director-General Dr. Tedros Adhanom Ghebreyesus said it was “dangerous” to think that the covid-19 epidemic would continue to decline.
“Last week, one person died every 44 seconds. Most of these deaths could have been prevented,” he said. “You’re probably tired of hearing me say the epidemic isn’t over, but I’ll keep saying it until it is,” he added.
Source: BFM TV