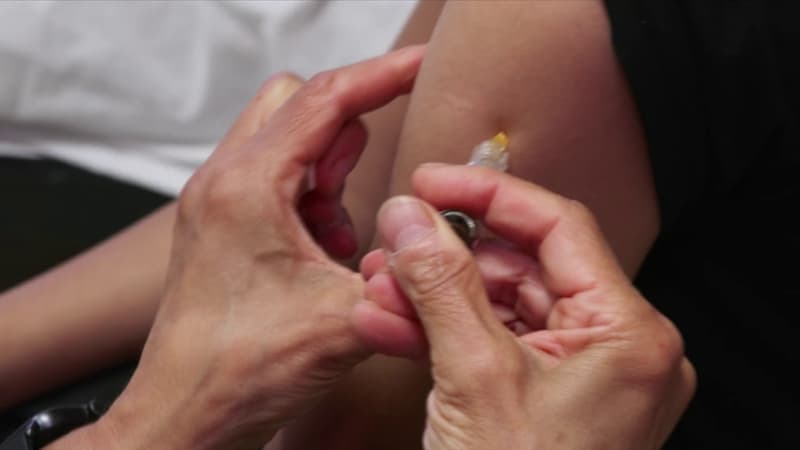
The Gardasil 9 vaccine against Human Papillomavirus (HPV), which has been the issue since 2023 of a vaccination campaign in universities, is still sure, said the drug agency on Thursday 21, emphasizing that no new risk had appeared since its previous balance sheets.
“All available data confirm that the vaccine is safe”, the National Medication Security Agency (ANSM), which has been reinforced by Gardasil 9 since the beginning of the university campaign in a statement.
Vaccination avoids up to 90% of HPV infections from cancer (uterus, anus, ent …). Since the 2023-2024 school year, fifth students can be vaccinated with total health insurance management, subject to the authorization of parents, in all public universities and volunteer private establishments.
“There is no new risk”
Almost 420,000 adolescents received at least one dose of vaccine at the University or in the city during the 2023/2024 campaign, according to the public Santé France (SPF).
“No new risk has been highlighted” from July to December 2024, according to the drug agency. “As during the previous period (January 2023-June of 2024), a new security signal has not been identified,” he added.
During the second half of 2024, 208 cases of undesirable effects were declared, including 55 as part of the university campaign. A large majority of these cases (166) had no seriousness, and 42 were classified serious, without death or at stake with the vital prognosis, according to the ANSM.
“The serious cases raised during the period are mainly consultations in the emergency room or hospitalizations after headache or abdominal pain, spontaneously resolved” but also discomfort, including “eight cases of discomforts that led to hospitalization,” said the agency.
Undesirable “rare and transient” effects “
“The known effects declared after a vaccination with Gardasil 9 are mainly reactions to the vaccine injection site (pain in the place of the bite, redness, pain and/or inflammation), headache, fatigue, fever, discomfort or even the brief loss of knowledge. These reactions appear shortly after vaccination and disappear quickly,” the ANS recalls in his press release.
People who receive the vaccine must be monitored for a quarter of an hour after injection to allow “preventing possible discomfort or syncopes, sometimes without announcing signs, especially among adolescents.”
“These reactions, although rare and transitory, can be accompanied by tremors or rigidity,” said the drug agency.
And, “in very rare cases, an anaphylactic reaction (allergic, editor’s note) can occur, as for any injectable vaccine,” he added, recommending that he systematically have adequate medical treatment.
After the death of a university student victim of a fall after the discomfort after vaccination during the 2023-2024 campaign, the drug agency recommended lengthening or establishing students in the field after vaccination. The next report on the pharmacovigilance of this vaccine will be published at the end of the year.
Source: BFM TV