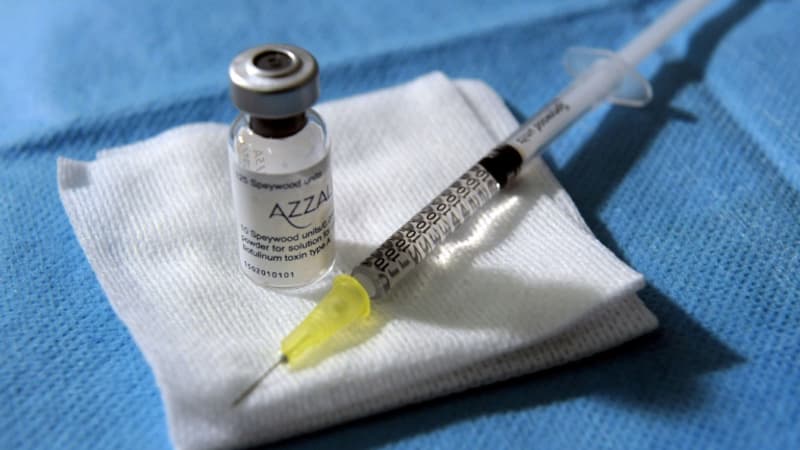
The Medicines Safety Agency (ANSM) again warned this Thursday, October 23, against illegal injections of botulinum toxin, better known as Botox, used to temporarily reduce wrinkles, after reporting new serious cases of botulism.
Since its last alert issued in February, the health authority reports in a press release that “three new serious cases” have been recorded in France following illegal injections of botulinum toxin, “carried out by unauthorized people.”
The affected patients presented “severe symptoms of botulism, in particular respiratory difficulties that required hospitalization in intensive care,” underlines the ANSM.
Serious risks outside the medical environment
Botulinum toxin is a medication that causes muscle relaxation, used both to treat certain muscular or neurological disorders and in aesthetic medicine.
It can cause a serious neurological disease, botulism, which can be fatal when used in uncontrolled doses, poorly administered or obtained from unauthorized products, reminds the ANSM.
Symptoms of the disease include blurred or double vision, difficulty breathing, difficulty speaking or swallowing.
These injections should be performed only by authorized healthcare professionals in a safe environment, such as doctors specialized in plastic, reconstructive and aesthetic surgery, dermatology, face and neck surgery, maxillofacial surgery and ophthalmology, with authorized medications, the safety and effectiveness of which have been evaluated, the anti-drug watchdog insists.
“The use of injections outside the medical environment presents significant risks, such as infections, skin necrosis, allergic reactions or even death,” says the ANSM.
Medical prescription requested
The institution had already warned about this phenomenon in February after the hospitalization of eight women who had presented serious symptoms of botulism, mainly related to an “overdose” during injections performed by “unqualified people” in a center in the Paris region between August and September 2024.
Reserved for professional use, botulinum toxin for aesthetic purposes is available in pharmacies or delivered directly by the manufacturer or its official supplier to a specialized doctor.
It must be subject to a medical prescription and its sale on the Internet or directly to the general public is prohibited.
Source: BFM TV